By Jake Ahern, Hugh D. Piggins, and Alan R. Champneys
Most people have at least a vague understanding of circadian rhythms. We experience them in our daily patterns of sleep and wake time; if we are fortunate, such rhythms coincide with the 24-hour fluctuation of ambient light. Many of us have also experienced the adverse effects of a disrupted circadian rhythm. Anyone who has traveled rapidly across multiple time zones—on transcontinental or transatlantic flights, for example—likely understands that disrupting the synchrony between the day-night and sleep-wake cycles can lead to numerous problems that range from cognitive deficits to digestive complications — many of which appear unrelated to the rhythm of ambient light. The seemingly disparate effects of jet lag and other timing issues, such as shift work, are connected by the far-reaching hold of the body’s circadian rhythm over almost every aspect of one’s physiology; body temperature fluctuations, hormone concentrations, and cognitive abilities are but a few additional examples of circadian-regulated processes. For example, numerous structures within the brain contain a functional circadian clock that facilitates daily variations in a plethora of central nervous system operations. These daily biological changes are not just limited to humans. Many forms of life—from bacteria to butterflies to bears—utilize evolved circadian clocks to anticipate the predictable rotation of planet Earth.
What exactly are these ubiquitous circadian rhythms? Broadly speaking, they are biological rhythms that repeat themselves approximately (circa-) every day (-dian). The circadian processes that we observe—like elevated levels of cortisol (the “stress hormone”) before we wake up—result from changes within the cells of the underlying tissues. Almost all cells in the mammalian body have their own little clocks that are made from genes and proteins. The components of these molecular clocks interact with one another such that their concentrations are locked into oscillations with periods of roughly 24 hours. This timekeeping mechanism, known as a transcription-translation feedback loop, is comprised of a negative feedback loop in which the activation of “clock” genes produces “clock” proteins; these same proteins then inhibit their own production by “turning off” their own genes. With various steps that add time delays and additional positive and negative feedback loops, the end result is a robust and accurate clock that quietly persists throughout most of the body’s cells.
Throughout the history of biological rhythms research, mathematical modeling has accompanied experimental techniques. Models of nonlinear oscillators and their interactions were the bread and butter of many physicists’ and applied mathematicians’ expertise in the 1970s, when circadian research was gaining popularity. The paucity of physiological mechanisms that underpin biological clock function led researchers to tune abstract models—such as phase oscillators or relaxation-type van der Pol oscillators—to 24 hours in order to study the interactions of different circadian rhythms. More detailed physiological understanding lead to more detailed models. Yet even before scientists discovered the core molecular components of the clock, Brian Goodwin’s seminal model described a negative feedback loop that would eventually serve as the core feature of all biological clocks [4].
Today, the symbiotic relationship between experimental developments and theoretical considerations is more vibrant than ever. Experiments inform models as much as simulations inform experiments, and the wealth of data analysis techniques for rhythmic time series helps us understand the implications of our experiments. During the 2021 SIAM Conference on Applications of Dynamical Systems (DS21), a three-part minisymposium about “The Dynamics of Circadian Oscillators: Mathematical Models beyond Kuramoto” explored the cutting-edge interface between circadian physiology and dynamical systems modeling.
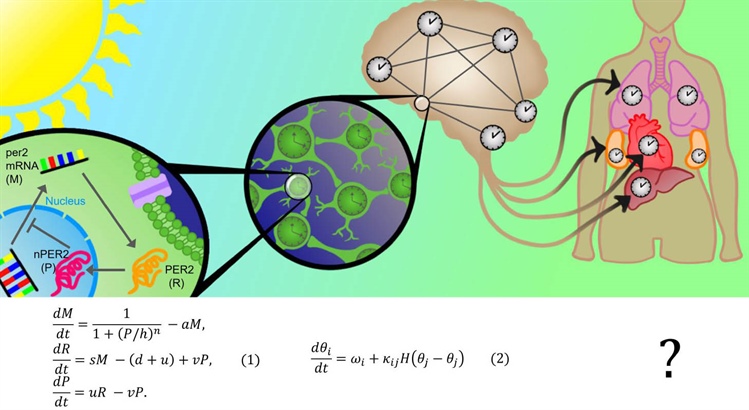
Figure 1. The mammalian brain contains many circadian clocks that can communicate with one another to form a distributed network. Activity in brain regions that contain these clocks may alter the clock network and the internal representation of time that it controls. A critical hub of this network is the suprachiasmatic nucleus (SCN), which receives light signals from the retina and synchronizes the network to the external cycle of day and night. The SCN itself is also a network of clock cells that are synchronized into a coherent clock by action potentials and gap junctions. A network of genes and proteins is present within each individual clock cell. The core oscillatory mechanism of the clocks is a negative feedback loop, such as the simplified per2 loop shown in \((1)\). The per2 gene is transcribed into per2 mRNA (\(M\)), which is translated into its protein product PER2 (\(R\)). Various biological processes transport PER2 into the nucleus nPER2 (\(P\)), where it inhibits the initial transcription step and closes the feedback loop. A plethora of mathematical models describe this process, with \((1)\) being among the simplest [6]. At a less granular level, coupled oscillator theory has greatly advanced our understanding of the clock cell network; even simple phase models like \((2)\) can reveal much about the cellular network’s structure and dynamics. At an even larger scale, an organism-wide circadian network likely exists. Multiple organs contain circadian clocks, and the SCN orchestrates at least some of them. The degree to which biological clocks interact with each other and with the brain’s circadian clocks is still unclear, and no qualitative understanding of an organism-wide clock network is evident (represented as the “?”). Figure courtesy of Jake Ahern.
Ozgur Akman (University of Exeter) addressed the simplest types of mathematical models in the field by discussing Boolean logic models of oscillating genetic networks. Traditionally, researchers model the components of a gene network—such as the one that underlies circadian rhythmicity—as a dynamical system. But this method quickly accumulates parameters, many of which are difficult to tune. As such, optimization of these models requires computational resources and large data sets. Akman and colleagues developed a Boolean approach that replaces the continuous oscillation of a molecule’s concentration with a simple oscillation between ON and OFF [1]. These simple models have far fewer parameters than traditional ordinary differential equation (ODE) models, and fitting them—especially when they are optimized with evolutionary algorithms—requires substantially less computational demand. Such “reduced models” hold up well against their more complicated ODE cousins; they correctly simulate the phase differences between various molecular components and can readily reproduce complex responses to light stimuli.
Hugh Piggins (University of Bristol) provided an overview of the current understanding of circadian rhythms. In recent decades, more and more studies have examined numerous tissues that display some sort of daily rhythmicity. These findings indicate that many parts of the body—especially the brain—possess tissues that act as their own clocks. Conventional wisdom had maintained that the “master clock” within the suprachiasmatic nucleus (SCN) of the hypothalamus is the intrinsic dominant pacemaker that forces the rhythmicity of “slave” clocks, and all such “slave” clocks must adhere to the beat of the SCN. However, discoveries of multiple nuclei in the brain that possess daily oscillations that are independent of the SCN and alter the SCN’s frequency allude to the exciting possibility of a distributed network of daily oscillators. The investigation of a network of coupled oscillators that are distributed throughout the brain and body will rely on both experimental advances and a consistent theoretical framework.
Jihwan Myung’s (Taipei Medical University) talk focused on the multitude of neuronal nuclei that possess a circadian rhythm but have varying degrees of synchrony between their cellular components. To create a tissue-level clock like the SCN, individual cellular rhythms must synchronize and transmit a coherent timing signal to the rest of the brain. Circadian clocks can use their intercellular synchrony as a form of information. For example, the SCN encodes information on day length in the phase difference between different cellular oscillators [7]. Furthermore, not only is desynchrony within clocks functionally relevant, but the level of synchrony between clocks may also encode information. Myung pointed out that a range of phase differences exist between all recently discovered circadian nuclei. His mapping of circadian clock phases in the brain may inspire a more thorough understanding of the circadian system.
The dorsal vagal complex (DVC) in the brainstem possesses a network of circadian oscillators that may also have functionally relevant phase differences. Jake Ahern’s (University of Bristol) presentation outlined a simple method to infer a minimal communication network between the DVC’s three constituent oscillators that relies on a Kuramoto-type framework and bifurcation analysis. A system of three juxtaposed, tissue-level oscillators—such as the ones in the DVC—is a rare occurrence in circadian biology. This system offers an ideal playground for the development of circadian network theories that we can readily investigate with standard experimental techniques.
In addition to building models that compliment experimental findings, researchers extensively utilize mathematical techniques to analyze rich data sets from physiological experiments. Bioluminescent reporters of clock gene activity lend insight into cellular and tissue rhythms and yield large data sets of rhythmic time series. Tanya Leise (Amherst College) has made significant contributions to the analysis of circadian time series data; during her DS21 talk, she discussed the ways in which practitioners can better account for biological noise and heterogeneity during analysis. One can feasibly apply Bayesian spectral analysis to time series data; such analysis may provide better estimates of properties like the phase and period of an oscillator, thus strengthening the link between the properties of a biological system and the mathematical models that describe it [2].
Mathematical techniques also help us understand the benefits of a better relationship with our internal rhythms. Casey Diekman (New Jersey Institute of Technology) added a new spin to a preexisting model of human circadian rhythm and found that the severity of desynchronization between internal and environmental rhythms (due to jet lag or shift work changes, for example) is reliant on the type of bifurcation that causes the desynchrony [3]. This bifurcation depends upon the light intensity, therefore illuminating an interesting link to possible therapeutic methods.
The relationship between abnormal sleep and circadian timing—which is increasingly important given the prevalence of artificial light in the modern world—was the focus of Andrew Phillips’ (Monash University) talk. Phillips explained that the physiological bases of both sleep and human circadian rhythms now exist in a single model that provides a framework to link observed phenotypes—such as “night owl” or “morning lark”—with the underlying physiology. Models that connect non-intrusive human measurements with circadian biology will be valuable tools during the development of individualized chronotherapeutic procedures. The recent increase in wearable technology and digital health monitoring has enabled the use of advanced data analysis techniques to estimate an individual’s internal time [5]. We can then use these measurements to provide treatments like drug delivery at optimal times.
The future of circadian biology research is bright. Our understanding of fundamental biological timekeeping is advancing, both in terms of our comprehension of the detailed physiology and our knowledge of biological oscillator dynamics. As we attempt to make sense of the large data sets that modern equipment can generate, quantitative techniques are more important than ever. These data sets also form an integral bridge between what we can measure and what we understand about the system behind the measurements. Future studies will heavily rely upon the fruitful symbiosis between the mathematics of oscillations and chronobiology. Remaining open questions—such as how a multitude of clocks arrange themselves into an organism-wide symphony of rhythms, or how we can use these rhythms to reconstruct a personal internal time—are intimately linked to close collaboration between the biological and mathematical sciences.
References
[1] Akman, O.E., Watterson, S., Parton, A., Binns, N., Millar, A.J., & Ghazal, P. (2012). Digital clocks: Simple Boolean models can quantitatively describe circadian systems. J. R. Soc. Interface, 9(74), 2365-2382.
[2] Cohen, A.L., Leise, T.L., & Welsh, D.K. (2012). Bayesian statistical analysis of circadian oscillations in fibroblasts. J. Theor. Biol., 314, 182-191.
[3] Creaser, J.L., Diekman, C.O., & Wedgwood, K.C.A. (2021). Entrainment dynamics organised by global manifolds in a circadian pacemaker model. Front. Appl. Math. Stat., 7.
[4] Gonze, D., & Ruoff, P. (2020). The Goodwin oscillator and its legacy. Acta Biotheoret., 69(4), 857-874.
[5] Kim, D.W., Zavala, E., & Kim, J.K. (2020). Wearable technology and systems modeling for personalized chronotherapy. Curr. Opin. Syst. Biol., 21, 9-15.
[6] Kurosawa, G., Mochizuki, A., & Iwasa, Y. (2002). Comparative study of circadian clock models, in search of processes promoting oscillation. J. Theor. Biol., 216(2), 193-208.
[7] Myung, J., & Pauls, J.D. (2018). Encoding seasonal information in a two-oscillator model of the multi-oscillator circadian clock. Eur. J. Neurosci., 48(8), 2718-2727.
 |
Jake Ahern is a Ph.D. student in computational neuroscience at the University of Bristol with an interest in dynamical systems modeling and biological oscillations. |
 |
Hugh D. Piggins is a circadian neuroscientist at the University of Bristol. His lab researches the organization of circadian oscillators in the brain. |
 |
Alan R. Champneys is a professor of applied nonlinear mathematics at the University of Bristol and a past chair of the SIAM Activity Group on Dynamical Systems. |